كربونات السيزيوم
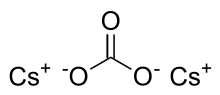
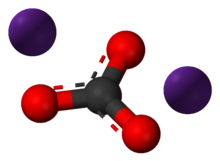
Caesium carbonate or cesium carbonate is a chemical compound with the chemical formula Cs
2CO
3. It is white crystalline solid. Caesium carbonate has a high solubility in polar solvents such as water, ethanol and DMF. Its solubility is higher in organic solvents compared to other carbonates like potassium carbonate and sodium carbonate, although it remains quite insoluble in other organic solvents such as toluene, p-xylene, and chlorobenzene. This compound is used in organic synthesis as a base.[2] It also appears to have applications in energy conversion.
| |
 Caesium, Cs Carbon, C Oxygen, O | |

| |
| الأسماء | |
|---|---|
| اسم أيوپاك المفضل
Dicaesium carbonate | |
أسماء أخرى
| |
| Identifiers | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| رقم EC |
|
PubChem CID
|
|
| UNII | |
| InChI | InChI={{{value}}} |
| SMILES | |
| الخصائص | |
| الصيغة الجزيئية | CCs2O3 |
| كتلة مولية | 325.8 g mol-1 |
| المظهر | white powder |
| الكثافة | 4.072 g/cm3 |
| نقطة الانصهار | |
| قابلية الذوبان في الماء | 2605 g/L (15 °C) |
| قابلية الذوبان في ethanol | 110 g/L |
| قابلية الذوبان في dimethylformamide | 119.6 g/L |
| قابلية الذوبان في dimethyl sulfoxide | 361.7 g/L |
| قابلية الذوبان في sulfolane | 394.2 g/L |
| قابلية الذوبان في methylpyrrolidone | 723.3 g/L |
| القابلية المغناطيسية | −103.6·10−6 cm3/mol |
| المخاطر | |
| نقطة الوميض | Non-flammable |
| مركبات ذا علاقة | |
أنيونات أخرى
|
Caesium bicarbonate |
كاتيونات أخرى
|
|
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
التحضير
Caesium carbonate can be prepared by thermal decomposition of caesium oxalate.[3] Upon heating, caesium oxalate is converted to caesium carbonate with emission of carbon monoxide.
- Cs
2C
2O
4 → Cs
2CO
3 + CO
It can also be synthesized by reacting caesium hydroxide with carbon dioxide.[3]
- 2 CsOH + CO
2 → Cs
2CO
3 + H
2O
التفاعلات الكيميائية
Caesium carbonate facilitates the N-alkylation of compounds such as sulfonamides, amines, β-lactams, indoles, heterocyclic compounds, N-substituted aromatic imides, phthalimides, and other similar compounds.[4] Research on these compounds has focused on their synthesis and biological activity.[5] In the presence of sodium tetrachloroaurate (Na[AuCl
4]), caesium carbonate is very efficient mechanism for aerobic oxidation of different kinds of alcohols into ketones and aldehydes at room temperature without additional polymeric compounds. There is no acid formation produced when primary alcohols are used.[6] The process of selective oxidation of alcohols to carbonyls had been quite difficult due to the nucleophilic character of the carbonyl intermediate.[5] In the past Cr(VI) and Mn(VII) reagents have been used to oxidize alcohols, however, these reagents are toxic and comparatively expensive. Caesium carbonate can also be used in Suzuki, Heck, and Sonogashira synthesis reactions. Caesium carbonate produces carbonylation of alcohols and carbaminationقالب:Cln of amines more efficiently than some of the mechanisms that have been introduced in the past.[7] Caesium carbonate can be used for sensitive synthesis when a balanced strong base is needed.
For energy conversion
Relatively effective polymer solar cells are built by thermal annealing of caesium carbonate. Caesium carbonate increases the energy effectiveness of the power conversion of solar cells and enhances the life times of the equipment.[8] The studies done on UPS and XPS reveal that the system will do less work due to the thermal annealing of the Cs
2CO
3 layer. Caesium carbonate breaks down into Cs
2O and Cs
2O
2 by thermal evaporation. It was suggested that, when Cs
2O combines with Cs
2O
2 they produce n-type dopes that supplies additional conducting electrons to the host devices. This produces a highly efficient inverted cell that can be used to further improve the efficiency of polymer solar cells or to design adequate multijunction photovoltaic cells.[9]
The nanostructure layers of Cs
2CO
3 can be used as cathodes for organic electronic materials due to its capacity to increase the kinetic energy of the electrons. The nanostructure layers of caesium carbonate had been probed for various fields using different techniques. The fields include such as photovoltaic studies, current-voltage measurements, UV photoelectron spectroscopy, X-ray photoelectron spectroscopy, and impedance spectroscopy. The n-type semiconductor produced by thermal evaporation of Cs
2CO
3 reacts intensively with metals like Al, and Ca in the cathode. This reaction will cut down the work the cathode metals.[10] Polymer solar cells based on solution process are under extensive studies due to their advantage in producing low cost solar cells. Lithium fluoride has been used to raise the power conversion efficiency of polymer solar cells. However, it requires high temperatures (> 500 degree), and high vacuum states raise the cost of production. The devices with Cs
2CO
3 layers have produced equivalent power conversion efficiency compared with the devices that use lithium fluoride.[8] Placing a Cs
2CO
3 layer in between the cathode and the light-emitting polymer improves the efficiency of the white OLED.
References
- ^ قالب:RubberBible62nd.
- ^ Sivik, Mark R.; Ghosh, Arun K.; Sarkar, Anindya (2001). "Cesium Carbonate". Encyclopedia of Reagents for Organic Synthesis. pp. 1–12. doi:10.1002/047084289X.rc049.pub2. ISBN 9780470842898.
- ^ أ ب E. L. Simons; E. J. Cairns; L. D. Sangermano (1966). "Purification and preparation of some caesium compounds". Talanta. 13 (2): 199–204. doi:10.1016/0039-9140(66)80026-7. PMID 18959868.
- ^ Mercedes, Escudero; Lautaro D. Kremenchuzky; a Isabel A. Perillo; Hugo Cerecetto; María Blanco (2010). "Efficient Cesium Carbonate Promoted N-Alkylations of Aromatic Cyclic Imides Under Microwave Irradiation". Synthesis. 2011 (4): 571. doi:10.1055/s-0030-1258398.
- ^ أ ب Babak, Karimi; Frahad Kabiri Estanhani (2009). "Gold nanoparticles supported on Cs2CO3 as recyclable catalyst system for selective aerobic oxidation of alcohols at room temperature". Chemical Communications. 5556 (55): 5555–5557. doi:10.1039/b908964k. PMID 19753355.
- ^ Lie, Liand; Guodong Rao; Hao-Ling Sun; Jun-Long Zhang (2010). "Aerobic Oxidation of Primary Alcohols Catalyzed by Copper Salts and Catalytically Active m-Hydroxyl-Bridged Trinuclear Copper Intermediate" (PDF). Advanced Synthesis & Catalysis. 352 (23): 2371–2377. doi:10.1002/adsc.201000456. Archived from the original (reprint) on 2014-02-01. Retrieved 2012-04-27.
- ^ Rattan, Gujadhur; D. Venkataraman; Jeremy T. Kintigh (2001). "Formation of aryl–nitrogen bonds using a soluble copper(I) catalyst" (PDF). Tetrahedron Letters. 42 (29): 4791–4793. doi:10.1016/s0040-4039(01)00888-7.
- ^ أ ب Jinsong, Huang; Zheng Xu; Yang Yang (2007). 2CO3.pdf "Low-Work-Function Surface Formed by Solution-Processed and Thermally Deposited Nanoscale Layers of Cesium Carbonate" (PDF). Advanced Functional Materials. 17 (19): 1966–1973. doi:10.1002/adfm.200700051. S2CID 44557096. Retrieved 2012-03-31.[dead link]
- ^ Hua-Hstien, Liao; Li-Min Chen; Zheng Xu; Gang Li; Yang Yang (2008). "Highly efficient inverted polymer solar cell by low temperature annealing of Cs2CO3 interlayer" (PDF). Applied Physics Letters. 92 (17): 173303. Bibcode:2008ApPhL..92q3303L. doi:10.1063/1.2918983.
- ^ Jen-Chun, Wang; Wei-Tse Weng; Meng-Yen Tsai; Ming-Kun Lee; Sheng-Fu Horng; Tsong-Pyng Perng; Chi-Chung Kei; Chih-Chieh Yuc; Hsin-Fei Meng. "Highly efficient flexible inverted organic solar cells using atomic layer deposited ZnO as electron selective layer". Journal of Materials.
Further reading
- Crich, David; Banerjee, Abhisek (2006). "Expedient Synthesis of syn-β-Hydroxy-α-amino acid derivatives: Phenylalanine, Tyrosine, Histidine and Tryptophan". J. Org. Chem. 71 (18): 7106–9. doi:10.1021/jo061159i. PMC 2621330. PMID 16930077.
- Gerard, Dijkstra; Wim H. Kruizinga; Richard M. Kellogg (1987). "An Assessment of the Causes of the "Cesium Effect"". J. Org. Chem. 52 (19): 4230. doi:10.1021/jo00228a015.
External links
- قالب:OrgSynth preps
- Caesium carbonate factsheet from Chemetall GmbH
- Material Safety Data Sheet Cesium Carbonate, 99.5%
| H2CO3 | He | |||||||||||||||||
| Li2CO3 | BeCO3 | B | C | N | O | F | Ne | |||||||||||
| Na2CO3 | MgCO3 | Al | Si | P | S | Cl | Ar | |||||||||||
| K2CO3 | CaCO3 | Sc | Ti | V | Cr | MnCO3 | FeCO3 | CoCO3 | NiCO3 | CuCO3 | ZnCO3 | Ga | Ge | As | Se | Br | Kr | |
| Rb2CO3 | SrCO3 | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag2CO3 | CdCO3 | In | Sn | Sb | Te | I | Xe | |
| Cs2CO3 | BaCO3 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl2CO3 | PbCO3 | Bi | Po | At | Rn | ||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||
| ↓ | ||||||||||||||||||
| La2(CO3)3 | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | ||||