ثلاثي أكسيد الزرنيخ
ثلاثي أكسيد الزرنيخ Arsenic trioxide هو مركب كيميائي له الصيغة As2O3 ، ويكون على شكل بلورات بيضاء. وهذا الأكسيد الهام تجارياً للزرنيخ هو السابق الرئيسي لمنتجات الزرنيخ الأخرى، بما فيها مركبات الزرنيخ العضوية. ويـُنتج منه نحو 50,000 طن سنوياً.[3] والكثير من استخداماته هي موضع جدل بسبب السمية العالية لمركبات الزرنيخ.

| |
| |
| الأسماء | |
|---|---|
| أسماء أخرى
Arsenic(III) oxide,
Arsenic sesquioxide, Arsenicum album, Arseneous oxide, Arseneous anhydride, White arsenic[1] | |
| Identifiers | |
| رقم CAS | |
3D model (JSmol)
|
|
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.014.075 |
| رقم EC |
|
PubChem CID
|
|
| رقم RTECS |
|
CompTox Dashboard (EPA)
|
|
| InChI | InChI={{{value}}} |
| SMILES | |
| الخصائص | |
| الصيغة الجزيئية | As2O3 |
| كتلة مولية | 197.841 g/mol |
| المظهر | White solid |
| الكثافة | 3.74 g/cm3 |
| نقطة الانصهار | |
| نقطة الغليان | |
| قابلية الذوبان في الماء | 2 g/100 ml (25°C) see text |
| قابلية الذوبان | soluble in dilute acids and alkalies, practically insoluble in organic solvents [2] |
| الحموضة (pKa) | 9.2 |
| البنية | |
| البنية البلورية | cubic (α)<180°C monoclinic (β) >180°C |
| الشكل الجزيئي | See Text |
| Dipole moment | Zero |
| الكيمياء الحرارية | |
| الإنتالپية المعيارية للتشكل ΔfH |
−657.4 kJ/mol |
| Standard molar entropy S |
? J.K–1.mol–1 |
| فارماكولوجيا | |
| رباط البروتينات | 75% bound |
| المخاطر | |
تبويب الاتحاد الاوروپي (DSD)
|
Very toxic (T+) Carc. Cat. 1 Dangerous for the environment (N) |
| توصيف المخاطر | R45, R28, R34, R50/53 |
| تحذيرات وقائية | S53, S45, S60, S61 |
| NFPA 704 (معيـَّن النار) | |
| الجرعة أو التركيز القاتل (LD, LC): | |
LD50 (الجرعة الوسطى)
|
14.6 mg/kg (rat, oral) |
| مركبات ذا علاقة | |
أنيونات أخرى
|
Arsenic trisulfide |
كاتيونات أخرى
|
Phosphorus trioxide Antimony trioxide |
ما لم يُذكر غير ذلك، البيانات المعطاة للمواد في حالاتهم العيارية (عند 25 °س [77 °ف]، 100 kPa). | |
| مراجع الجدول | |
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
الخواص
- ينحل ثالث أكسيد الزرنيخ بالماء والمحلات الأخرى بصعوبة، لكنه ينحل في الأحماض والقلويات مشكلاً الأملاح الموافقة حسب المعادلات:
- As2O3 + 6NaOH → 2Na3AsO3 + 3H2O
- As2O3 + 6HCl → 2AsCl3 + 3H2O
- يوجد ثالث أكسيد الزرنيخ بشكلين حسب البنية البلورية له، إحداهما مكعبة وهي ثابتة عند درجات الحرارة العادية، وأخرى أحادية الميل تكون ثابتة عند درجات حرارة فوق 221°س.
التحضير
- يوجد أكسيد الزرنيخ الثلاثي في الطبيعة.
- يحضر مخبرياً إما من حرق فلز الزرنيخ بالهواء حسب المعادلة:
- 2As + 3/2O2 → As2O3
- يحضر أكسيد الزرنيخ الثلاثي صناعياً من تحميص معدن الزرنيخ الذي يوجد غالباً على شكل كبريتيد مع فلز الحديد حسب المعادلة:
- 2FeAsS + 5O2 → Fe2O3 + As2O3 + 2SO2
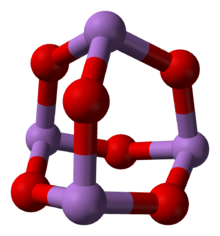
البنية
(cubic) |
(monoclinic) |
(monoclinic) |
الاستخدامات
- يعد أكسيد الزرنيخ الثلاثي مصدراً لإنتاج جميع مركبات الزرنيخ الأخرى.
Medical
Arsenic trioxide is indicated in combination with tretinoin for treatment of adults with newly-diagnosed low-risk acute promyelocytic leukemia whose acute promyelocytic leukemia is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression; and for induction of remission and consolidation in patients with acute promyelocytic leukemia who are refractory to, or have relapsed from, retinoid and anthracycline chemotherapy, and whose acute promyelocytic leukemia is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.[4][5][6]
Arsenic trioxide is used to treat a type of cancer known as acute promyelocytic leukemia (APL).[7] It may be used both in cases that are unresponsive to other agents, such as all-trans retinoic acid (ATRA) or as part of the initial treatment of newly diagnosed cases.[7] This initial treatment may include combination therapy of arsenic trioxide with all-trans retinoic acid (ATRA).[8]
Effectiveness appears similar to Realgar/Indigo naturalis, which can be taken by mouth and is less expensive but is less available.[9] It works by encouraging the proteosome breakdown of retinoic acid receptor alpha, by moving the protein on to the nuclear matrix and increasing ubiquitination.[10]
In the 1970s, Chinese researcher Zhang Tingdong and colleagues discovered this use.[11] It was approved for leukemia treatment in the United States in 2000.[12] University of Hong Kong developed a liquid form of arsenic trioxide that can be given by mouth.[13] Organoarsenic compounds, such as feed additives (roxarsone) and medication (neosalvarsan), are derived from arsenic trioxide.[بحاجة لمصدر]
Manufacturing
Industrial uses include usage as a precursor to forestry products, in colorless glass production, and in electronics.[3] Being the main compound of arsenic, the trioxide is the precursor to elemental arsenic, arsenic alloys, and arsenide semiconductors. Bulk arsenic-based compounds sodium arsenite and sodium cacodylate are derived from the trioxide.[بحاجة لمصدر]
A variety of applications exploit arsenic's toxicity, including the use of the oxide as a wood preservative. Copper arsenates, which are derived from arsenic trioxide, are used on a large scale as a wood preservative in the U.S. and Malaysia, but such materials are banned in many parts of the world. This practice remains controversial.[3] In combination with copper(II) acetate, arsenic trioxide gives the vibrant pigment known as Paris green used in paints and as a rodenticide. This application has been discontinued.[بحاجة لمصدر]
Alternative medicine
Despite the well known toxicity of arsenic, arsenic trioxide was used in traditional Chinese medicine, where it is known as pi-shuang (صينية: 砒霜؛ پنين: pīshuāng؛ حرفياً: 'arsenic frost'�). In homeopathy, it is called arsenicum album. Some discredited patent medicines, e.g., Fowler's solution, contained derivatives of arsenic oxide.[14]
الاستخدامات الطبية
الانتاج والتواجد الطبيعي
Arsenic trioxide can be generated via routine processing of arsenic compounds including the oxidation (combustion) of arsenic and arsenic-containing minerals in air. Illustrative is the roasting of orpiment, a typical arsenic sulfide ore.
- 2 As 2S 3 + 9 O 2 → 2 As 2O 3 + 6 SO 2
Most arsenic oxide is, however, obtained as a volatile by-product of the processing of other ores. For example, arsenopyrite, a common impurity in gold- and copper-containing ores, liberates arsenic trioxide upon heating in air. The processing of such minerals has led to numerous cases of poisonings,[15] and after the mine is closed, the leftover trioxide waste will present environmental hazard (as was the case with the Giant Mine, for example). Only in China are arsenic ores intentionally mined.[3]
In the laboratory, it is prepared by hydrolysis of arsenic trichloride:[16]
- 2 AsCl
3 + 3 H
2O → As
2O
3 + 6 HCl
As 2O 3 occurs naturally as two minerals, arsenolite (cubic) and claudetite (monoclinic). Both are relatively rare secondary minerals found in oxidation zones of As-rich ore deposits.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
السمية
Arsenic trioxide is readily absorbed by the digestive system. Ingestion of as little as 0.1 grams can be fatal.[3]
Chronic arsenic poisoning is known as arsenicosis. This disorder affects workers in smelters, in populations whose drinking water contains high levels of arsenic (0.3–0.4 ppm), and in patients treated for long periods with arsenic-based pharmaceuticals. Long-term ingestion of arsenic trioxide either in drinking water or as a medical treatment can lead to skin cancer. Reproductive problems (high incidences of miscarriage, low birth weight, congenital deformations) have also been indicated in one study of women exposed to arsenic trioxide dust as employees or neighbours of a copper foundry.
In the U.S., the OSHA 1910.1018 occupational permissible exposure limit for inorganic arsenic compounds in breathing zone air is 0.010 mg/m3.
المصادر
Taschenbuch chemische Substanzen, Willmes, Verlag Harri Deutsch, ISBN 3-8171-1662-4
الهامش
- ^ Shakhashiri BZ, "Chemical of the Week: Arsenic", University of Wisconsin-Madison Chemistry Dept.
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ^ أ ب ت ث ج Sabina C. Grund, Kunibert Hanusch, Hans Uwe Wolf "Arsenic and Arsenic Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, VCH-Wiley, 2008, Weinheim.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةTrisenox FDA label - ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةTrisenox EPAR - ^ "Drug Approval Package: Trisenox (Arsenic Trioxide) NDA #21-248". U.S. Food and Drug Administration (FDA). 12 July 2001. Archived from the original on 3 February 2024. Retrieved 3 February 2024.
- ^ أ ب خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةAHFS2019 - ^ Zhu J, Chen Z, Lallemand-Breitenbach V, de Thé H (September 2002). "How acute promyelocytic leukaemia revived arsenic". Nature Reviews. Cancer. 2 (9): 705–713. doi:10.1038/nrc887. PMID 12209159. S2CID 2815389.
- ^ Howard SC. "Proposal for the inclusion of arsenic therapies in the WHO Model List of Essential Medicines for the treatment of acute promyelocytic leukemia" (PDF). WHO. Archived from the original (PDF) on 9 March 2022. Retrieved 15 November 2019.
- ^ Zhu J, Koken MH, Quignon F, Chelbi-Alix MK, Degos L, Wang ZY, Chen Z, de Thé H (April 1997). "Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia". Proceedings of the National Academy of Sciences of the United States of America. 94 (8): 3978–3983. Bibcode:1997PNAS...94.3978Z. doi:10.1073/pnas.94.8.3978. PMC 20553. PMID 9108090.
- ^ Rao Y, Li R, Zhang D (June 2013). "A drug from poison: how the therapeutic effect of arsenic trioxide on acute promyelocytic leukemia was discovered". Science China Life Sciences. 56 (6): 495–502. doi:10.1007/s11427-013-4487-z. PMID 23645104.
- ^ Bian Z, Chen S, Cheng C, Wang J, Xiao H, Qin H (2012). "Developing new drugs from annals of Chinese medicine". Acta Pharmaceutica Sinica B. 2: 1–7. doi:10.1016/j.apsb.2011.12.007.
- ^ Au WY, Kumana CR, Kou M, Mak R, Chan GC, Lam CW, Kwong YL (July 2003). "Oral arsenic trioxide in the treatment of relapsed acute promyelocytic leukemia". Blood. 102 (1): 407–408. doi:10.1182/blood-2003-01-0298. PMID 12814916.
- ^ Gibaud S, Jaouen G (2010). "Arsenic-Based Drugs: From Fowler's Solution to Modern Anticancer Chemotherapy". Medicinal Organometallic Chemistry. Topics in Organometallic Chemistry. Vol. 32. pp. 1–20. Bibcode:2010moc..book....1G. doi:10.1007/978-3-642-13185-1_1. ISBN 978-3-642-13184-4.
- ^ "Giant Mine – Northwest Territories Region – Indian and Northern Affairs Canada". Archived from the original on 27 June 2006. Retrieved 28 August 2007.
- ^ خطأ استشهاد: وسم
<ref>غير صحيح؛ لا نص تم توفيره للمراجع المسماةBrauer
وصلات خارجية
- Case Studies in Environmental Medicine: Arsenic Toxicity
- IARC Monograph – Arsenic and Arsenic Compounds
- International Chemical Safety Card 0378
- NIOSH Pocket Guide to Chemical Hazards
- NTP Report on Carcinogens – Inorganic Arsenic Compounds
- Use of Arsenic Trioxide in Multiple Myeloma Treatment
- The use of Arsenic trioxide in medicine.
- قالب:Inrs
- Institute of Chemistry Austria, speciallised on arsenic and various arsenic compounds
